Starting YIMMUGO®
Dosing and administration
Well-tolerated dosing and infusion rate escalation1
RECOMMENDED DOSAGE1,2
infusion rate:
First Infusion
Subsequent Infusions
YIMMUGO delivers reliable infusions without compromising tolerability1
- No infusion-related adverse events above 4.0 mL/kg/h1
- Average infusion time decreased by 52 minutes and 71 minutes as the infusion rate increased1*
- The majority of infusions were completed without interruption or infusion rate reduction1
- 4 patients (6%) modified or interrupted 4 infusions (0.43%) due to TEAEs
THE YIMMUGO DIFFERENCE
YIMMUGO® Dosing Calculator for Primary Immunodeficiencies (PI)
Select the type of infusion you want to calculate:
| Total Dose | Total Infusion Volume | Initial Infusion Rate 0.5 mg/kg/min (0.005 mL/kg/min) | Maximum Infusion Rate 3.0 mg/kg/min (0.03 mL/kg/min) |
|---|---|---|---|
| g | mL | mg/min or mL/min | mg/min or mL/min |
| Total Dose | Total Infusion Volume | Initial Infusion Rate 0.5 mg/kg/min (0.005 mL/kg/min) | Maximum Infusion Rate 13.0 mg/kg/min (0.13 mL/kg/min) |
|---|---|---|---|
| g | mL | mg/min or mL/min | mg/min or mL/min |
Ordering YIMMUGO
YIMMUGO is available through a limited distribution network
Distributor
Specialty Pharmacy
-
Accredo Health Group
1-866-820-4844 -
Advanced Infusion Care (AIC)
1-877-443-4006 -
AOM Infusion
1-800-746-9089 -
AvevoRx
1-877-283-8679 -
BioMatrix Specialty Pharmacy, LLC
1-877-567-8087 -
Clinical Specialty Infusions of Dallas, LLC
1-833-569-1005 -
CVS Health®
1-866-899-1661 -
Elevance Health
-
InfuCare Rx
1-877-828-3940 -
KabaFusion, LLC
1-877-577-4844 -
Nufactor, Inc.
1-800-323-6832 -
Option Care Health®
1-877-974-4844 -
Optum Infusion Pharmacy
1-855-427-4682 -
PromptCare Home Infusion, LLC
1-866-776-6782 -
Soleo Health, Inc.
1-844-575-1515 -
Superior Biologics
1-855-494-3121 -
Vital Care Infusion Services, LLC’s Franchise Affiliates
1-800-447-4905
YIMMUGO is made to fit your patient’s needs2
THE YIMMUGO DIFFERENCE
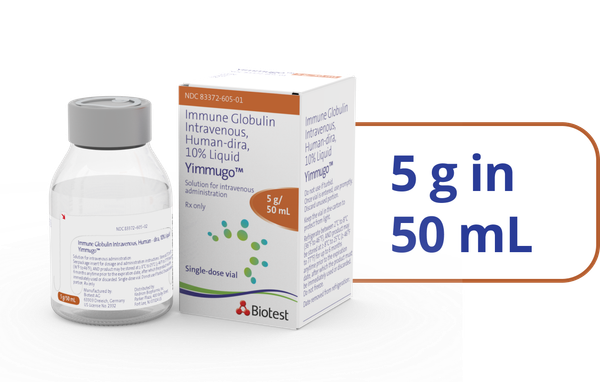
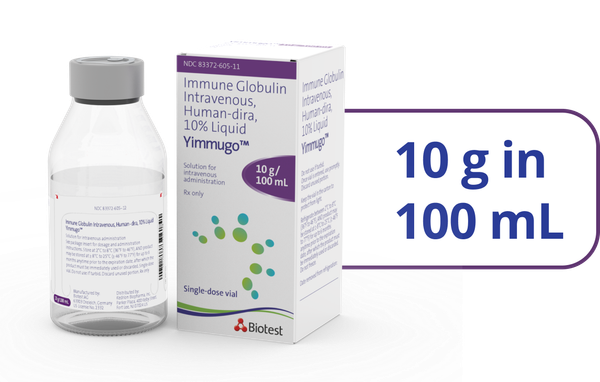
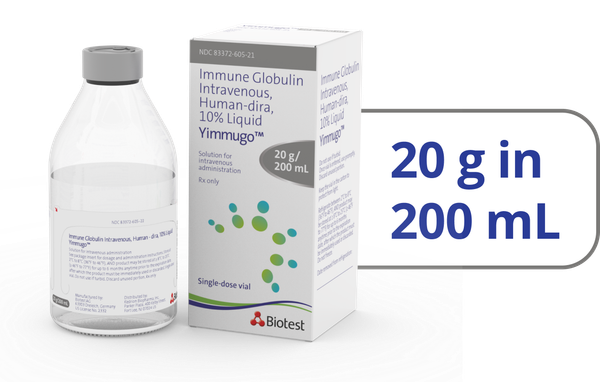
- *Of the 67 patients studied in the clinical trial, 12 patients received YIMMUGO on a Q3W schedule and 55 patients on a Q4W schedule. The Q3W schedule group experienced decreased infusion times from a median of 174 minutes (infusion 1) to 122 minutes (last protocol-defined infusion) and from a median of 165 minutes to 94 minutes in the Q4W schedule group.1
- Q3W, every 3 weeks; Q4W, every 4 weeks; TEAE, treatment-emergent adverse event.
Customer Service
Would you like to get in touch with us?
The Kedrion customer service team is here to help
Medical Information
General Information
Sign up to receive more information about YIMMUGO
INDICATIONS AND USAGE
YIMMUGO® (immune globulin intravenous, human – dira) is a 10% immune globulin (Ig) liquid indicated for the treatment of primary humoral immunodeficiency in patients 2 years of age and older.
IMPORTANT SAFETY INFORMATION
WARNING: THROMBOSIS, RENAL DYSFUNCTION, and ACUTE RENAL FAILURE
- Thrombosis may occur with Ig intravenous (IGIV) products, including YIMMUGO.
- Renal dysfunction, acute renal failure, osmotic nephrosis, and death may occur with the administration of IGIV products in predisposed patients. Renal dysfunction and failure occur more commonly in patients receiving IGIV products containing sucrose. YIMMUGO does not contain sucrose.
- For patients at risk of thrombosis, renal dysfunction, or renal failure, administer YIMMUGO at the minimum dose and infusion rate practicable. Ensure adequate hydration in patients before administration. Monitor for signs and symptoms of thrombosis and assess blood viscosity in patients at risk for hyperviscosity.
CONTRAINDICATIONS
YIMMUGO is contraindicated in patients who have had an anaphylactic or severe systemic reaction to the administration of human Ig and in patients with immunoglobulin A (IgA) deficiency who have antibodies against IgA and a history of hypersensitivity.
WARNINGS AND PRECAUTIONS
Severe hypersensitivity reactions, including anaphylaxis, have been reported after administration. In case of hypersensitivity, discontinue infusion immediately and institute appropriate treatment. YIMMUGO contains ≤300 mcg/mL of IgA. Patients with known antibodies to IgA may be at greater risk.
Hemolysis that is either intravascular or due to enhanced red blood cell sequestration can develop subsequent to IGIV treatment. Risk factors for hemolysis include high doses and non-O blood group. Monitor patients for hemolysis.
Renal failure: Monitor renal function, including blood urea nitrogen (BUN) and serum creatinine, and urine output in patients at risk of developing acute renal failure.
Hyperproteinemia, increased serum viscosity, and hyponatremia may occur in patients receiving IGIV treatment, including YIMMUGO.
Aseptic meningitis syndrome may occur in patients receiving IGIV treatment, especially with high doses or rapid infusion.
Transfusion-related acute lung injury: Monitor patients for pulmonary adverse reactions.
Transmissible infectious agents: YIMMUGO is made from human plasma and may contain infectious agents, eg, viruses and, theoretically, the Creutzfeldt-Jakob disease agent.
Interference with laboratory tests: After infusion of Ig, transitory rise of various passively transferred antibodies in the blood may yield positive serological results, with potential for misleading interpretation.
ADVERSE REACTIONS
The most common adverse reactions occurring in ≥5% of patients were headache, upper respiratory tract infections, fatigue, nausea, and increased blood pressure.
To report SUSPECTED ADVERSE REACTIONS, contact Kedrion Biopharma Inc. at 1-855-3KDRION (1-855-353-7466) or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch.
Please see full Prescribing Information for complete prescribing details, including Boxed Warning.
